Introduction:
Acalabrutinib is a highly selective, irreversible, next-generation, Bruton tyrosine kinase inhibitor (BTKi) approved for the treatment of patients with chronic lymphocytic leukemia (CLL). Acalabrutinib and ibrutinib are the only BTKis approved for the treatment of CLL, although acalabrutinib's minimal off-target effects may offer additional efficacy and safety benefits. ELEVATE-TN, a 3-arm, open-label, randomized, controlled trial (N=535 patients), found that acalabrutinib (as monotherapy or in combination with obinutuzumab) had superior efficacy and safety compared with chlorambucil plus obinutuzumab (C+O) for the frontline treatment of patients with CLL who were considered ineligible for fludarabine-based treatment because of age and comorbidity status. Countries such as the United Kingdom (UK) do not currently reimburse BTKi costs when used for the frontline treatment of CLL, except for patients with 17p deletions/TP53 mutations. Payers in these markets require evidence of cost-effectiveness, relative to the standard-of-care chemoimmunotherapy, for BTKi costs to be reimbursed in a broader frontline population. Therefore, the objective of this study is to evaluate the cost-effectiveness-from a UK National Health Service perspective-of acalabrutinib monotherapy compared to C+O in the frontline treatment of patients with CLL who are considered ineligible for fludarabine-based treatment.
Methods:
A semi-Markov model was developed, comprised of 3 health-state selections: progression-free survival (PFS), progressed disease, and death. ELEVATE-TN patient-level data were used to fit parametric survival functions to time-to-progression, preprogression-death, and postprogression-survival curves over a lifetime horizon of 30 years. Age- and gender-matched general population mortality rates were applied to ensure that the mortality rate in the modeled population never fell below that of the general population. Health-state utility data using EQ-5D scores were derived from published literature. Cost inputs included drug costs (list prices only), administration costs, subsequent-treatment costs, and adverse-event costs, and were sourced from relevant literature, including the British National Formulary, national reference costs, and UK CLL health technology assessment submissions. Subsequent treatment with ibrutinib was assumed in patients who experienced disease progression after C+O treatment, while patients who received acalabrutinib and had disease progression received rituximab in combination with venetoclax or bendamustine. Clinical validation was sought on model assumptions. A 3.5% discount rate was applied to both costs and outcomes, and an incremental cost-effectiveness ratio (ICER) of ≤ £30 000 per quality-adjusted life-year (QALY) was considered cost-effective.
Results:
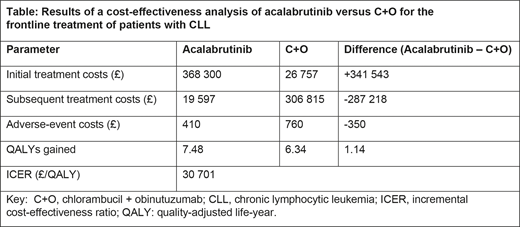
Over a 30-year lifetime horizon, treatment with acalabrutinib monotherapy resulted in a gain of 1.14 QALYs versus C+O, while incurring incremental costs of £34 974, resulting in an ICER of £30 701 per QALY (Table). While acalabrutinib treatment resulted in higher treatment costs than C+O because of the longer duration of treatment from improved PFS, these costs were partially offset by lower subsequent treatment costs and fewer adverse events compared with C+O. The key model drivers included the proportion of patients who received subsequent treatment with ibrutinib or venetoclax and the BTKi list price.
Conclusions:
Our analysis found that in a UK setting, acalabrutinib monotherapy was marginally cost-effective when compared with chemoimmunotherapy. This assessment of acalabrutinib provides useful information to payers for decision making among the frontline treatment options for the patient with CLL who is not eligible for fludarabine-based treatment.
Munir:F. Hoffmann-La Roche: Consultancy, Other: Medical writing support, furnished by Scott Battle, PhD, of Health Interactions, was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland; Alexion: Honoraria. Gaitonde:AstraZeneca: Current Employment, Other: stocks and share. Waweru:AstraZeneca: Current Employment, Other: stocks and share.
Author notes
Asterisk with author names denotes non-ASH members.